New England Journal of Medicine study shows high levels of protection in disproportionately affected populations
A study published in the New England Journal of Medicine involving data from nearly 200 hospitals around the United States shows that 2-dose COVID-19 vaccinations are highly effective at preventing hospitalizations, emergency department visits, and intensive care admissions due to the virus. The real-world evidence gathered from electronic health records (EHRs) demonstrates that the vaccines provide high levels of protection for populations disproportionately affected by the virus, including older adults and minorities.
The U.S. Centers for Disease Control and Prevention (CDC) collaborated with six U.S. healthcare systems plus the Regenstrief Institute, to create the VISION network to assess COVID-19 vaccine effectiveness. Regenstrief, Columbia University Irving Medical Center, HealthPartners, Intermountain Healthcare, Kaiser Permanente Northern California, Kaiser Permanente Northwest and University of Colorado all contributed hospitalization and ICU data for patients older than 50 from a total of 187 hospitals, in addition to data from emergency departments and urgent care clinics. The data covered 45,000 medical encounters.
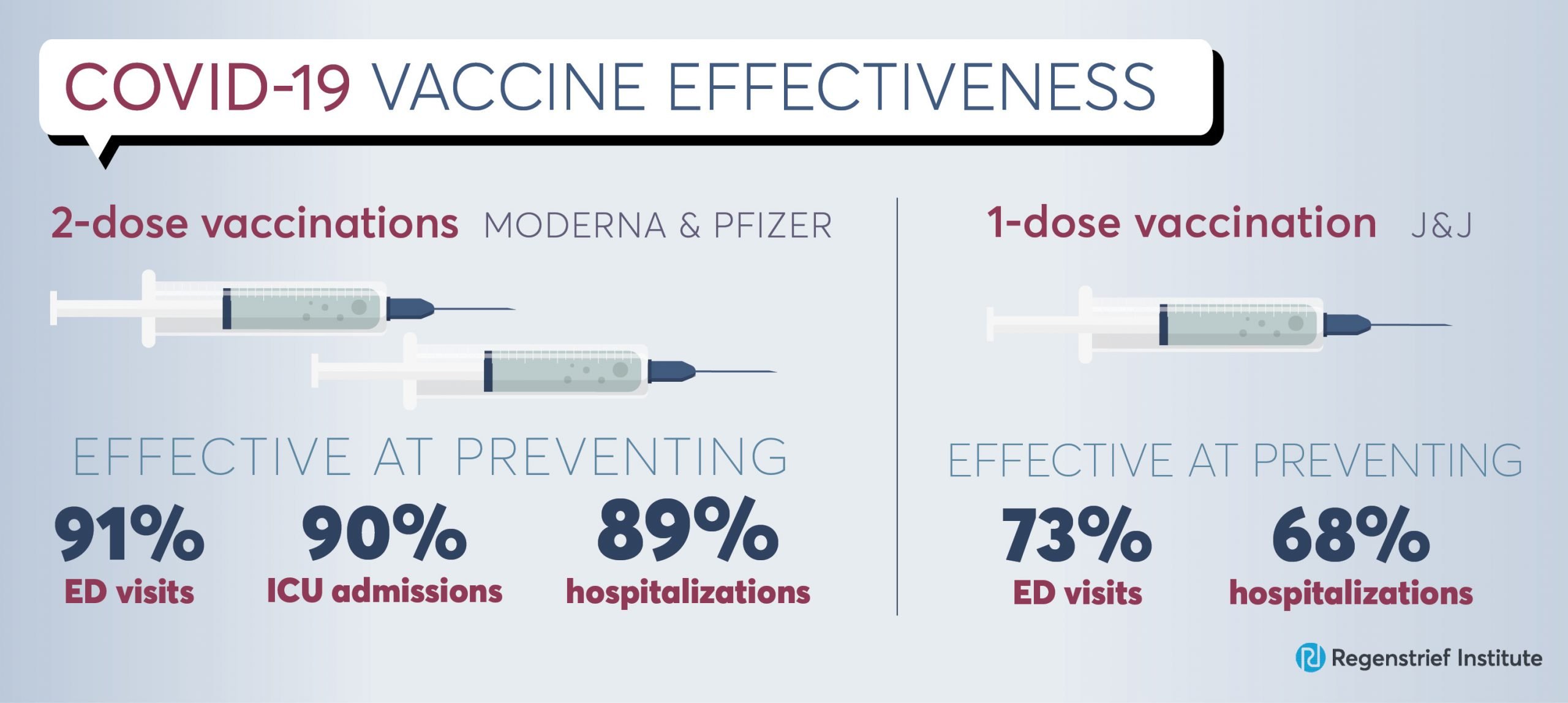
Data analysis showed 2-dose mRNA vaccination (Moderna and Pfizer) was:
- 89 percent effective at preventing COVID-19 hospitalizations
- 91 percent effective at preventing COVID-19 emergency department or urgent care visits
- 90 percent effective at preventing COVID-19 intensive care unit admission
The effectiveness was significantly lower in individuals who received only the first dose of the two shot-vaccination.
“This study confirms that these vaccines are highly effective,” said lead author Mark Thompson, PhD, a member of the CDC COVID-19 Response Team. “They offer significant protections for people older than 85, people with chronic medical conditions, as well as Black and Hispanic adults. All are groups who have been hit particularly hard by this disease. We hope this information will convince more people to get vaccinated to protect not only themselves but their community.”
This study was also one of the first to look at the effectiveness of the single-dose Johnson and Johnson vaccine. It was found to be 73 percent effective against emergency department and urgent care visits, and 68 percent against hospitalizations. However, the authors note the smaller sample size may affect the precision of these estimates and state that more data is needed.
“This real-world evidence corroborates the results of clinical trials and provides even more confidence in the vaccines,” said paper author Shaun Grannis, M.D., M.S., Regenstrief vice president for data and analytics and Indiana University School of Medicine professor of family medicine. “This study is an excellent example of how EHR data can be leveraged for public health, and how collaboration between health entities can provide new and beneficial insights.”
“Effectiveness of COVID-19 Vaccines in Preventing Ambulatory and Inpatient Care” is published in the New England Journal of Medicine. This study was funded by the Centers for Disease Control and Prevention through contract 75D30120C07986 to Westat, Inc. and contract 75D30120C07765 to Kaiser Foundation Hospitals.
In addition to Dr. Grannis, Regenstrief Director of Public Health Informatics Brian Dixon, PhD, MPA, was also an author on the paper along with Regenstrief affiliates William F. Fadel, PhD, and Nimish Ramesh Valvi, DrPH, MBBS, MPH.
Other authors are Edward Stenehjem, M.D., MSc of InterMountain Healthcare; Sarah W. Ball, ScD, M.S. of Westat; Allison L. Naleway, PhD of Kaiser Permanente Northwest; Toan C. Ong, PhD of University of Colorado; Malini B. DeSilva, M.D., MPH of Health Partners Institute; Karthik Natarajan, PhD of Columbia University; Catherine H. Bozio, PhD, MPH of the CDC COVID-19 Response Team; Edwin Lewis, MPH of Kaiser Permanente Northern California; Kristin Dascomb, M.D., PhD of Intermountain Healthcare; Rebecca J. Birch, MPH of Westat; Stephanie A. Irving, MHS of Westat; Suchitra Rao, M.D. of the University of Colorado; Elyse Kharbanda, M.D., MPH of HealthPartners Institute for Education and Research; Jungmi Han, B.S. of Columbia University; Sue Reynolds, PhD, M.S., MPH of the CDC COVID-19 Response Team; Kristin Goddard, MPH of the Kaiser Permanente Vaccine Study Center; Nancy Grisel, MPP of Intermountain Healthcare; Matthew E. Levy, PhD of Westat; Jill Ferdinands, PhD of the CDC COVID-19 Response Team; Bruce Fireman, PhD of Kaiser Permanente; Julie Arndorfer, MPH of Intermountain Healthcare; Elizabeth A. Rowley, DrPH, M.S. of Westat; Palak Patel, MBBS, MPH of the CDC COVID-19 Response Team; Ousseny Zerbo, PhD of the Kaiser Permanente Vaccine Study Center; Eric P. Griggs, MPH of the CDC COVID-19 Response Team; Rachael M. Porter, MPH of the CDC COVID-19 Response Team; Maria Demarco of Westat; Lenee Blanton, MPH of the CDC COVID-19 Response Team; Andrea Steffens, MPH of the CDC COVID-19 Response Team; Yan Zhuang, PhD, M.S. of Westat; Natalie Olson, MPH of CDC COVID-19 Response Team; Michelle Barron, M.D. of the University of Colorado; Patricia Shifflett, R.N., M.S. of Westat; Stephanie Schrag, DPhil of CDC COVID-19 Response Team; Jennifer Verani, M.D., MPH of CDC COVID-19 Response Team; Alicia Fry, M.D., MPH of CDC COVID-19 Response Team; Manjusha Gaglani, MBBS of Baylor Scott & White Health and Texas A&M University; Eduardo Azziz-Baumgartner, M.D., MPH of CDC COVID-19 Response Team and Nicola P. Klein, M.D., PhD of the Kaiser Permanente Vaccine Study Center.
About Regenstrief Institute
Founded in 1969 in Indianapolis, the Regenstrief Institute is a local, national and global leader dedicated to a world where better information empowers people to end disease and realize true health. A key research partner to Indiana University, Regenstrief and its research scientists are responsible for a growing number of major healthcare innovations and studies. Examples range from the development of global health information technology standards that enable the use and interoperability of electronic health records to improving patient-physician communications, to creating models of care that inform practice and improve the lives of patients around the globe.
Sam Regenstrief, a nationally successful entrepreneur from Connersville, Indiana, founded the institute with the goal of making healthcare more efficient and accessible for everyone. His vision continues to guide the institute’s research mission.
About Shaun Grannis, M.D., M.S.
In addition to his role as the vice president of data and analytics at Regenstrief Institute, Shaun Grannis, M.D., M.S., is the Regenstrief Chair in Medical Informatics and a professor of family medicine at Indiana University School of Medicine.